目前,金属锂电池的商业化应用仍面临诸多挑战:①电池循环过程中,易于形成锂枝晶,带来电池短路风险;②锂负极与电解液发生副反应,生成不稳定的固体电解质界面(SEI)膜;③充放电过程中严重的体积效应,造成电极结构完整性受损;④不断积累电化学失活的死锂,导致电池快速失效。
围绕这些问题,研究者们开展了大量研究,并取得了一系列进展,通过三维亲锂骨架设计,合金负极制备,固态电解质开发,SEI膜结构组成调控等手段有效抑制了枝晶的产生,并构筑稳定的SEI膜,并在一定程度上减少死锂的产生和积累。然而,深入研究发现表面死锂的产生和积累难以被彻底杜绝,死锂依旧是限制金属锂电池性能和寿命的关键难题。
想要有效根治死锂问题,首先要对死锂的结构组成、物化性质以及形成机制有一定的认识。然而,死锂是一种不稳定的含锂物质,难以通过一般的表征技术对其进行深度剖析,缺乏了基础认识也就难以提出解决死锂问题的靶向策略。基于作者及合作者在死锂研究方面的近期成果,本文首先将阐明死锂的结构组成及其形成的微观机制;随后将介绍并评述一些关键新型表征技术在死锂研究中的应用,并着重探讨解决死锂难题的有效策略,包括多功能骨架稳定体相及界面,人工界面保护层,死锂激活,以及固态电解质工程等;最后对动态死锂难题解决,以及金属锂电池实用化进程推进提出展望。
在先进表征技术的协助下,研究者们对于死锂有了一定的基本认识。死锂的形成与锂的沉积溶解行为,以及SEI膜的性质密切相关,因而解决死锂问题有两种不同的考虑:①死锂抑制策略。通过亲锂骨架、压力场等调控锂的沉积形貌,抑制高迂曲度的锂枝晶的生长,或者是构筑稳定的表面钝化层,抑制电解液对锂的腐蚀,并促进均匀退锂。②死锂转化利用策略。通过特定的物质将死锂进行转化、重储和再利用,或是在死锂内部原位搭建外源性导电网络,重新建立死锂和电极的电子通路,使得死锂可以被再次利用。基于此,研究者们开发了一系列策略来尝试解决死锂难题。
3.1 功能骨架稳定负极体相结构及界面
锂在导电骨架(如纯碳)中依旧会生长枝晶,形成死锂,功能化骨架设计可以进一步提升其对锂负极的稳定作用,例如亲锂骨架。亲锂骨架由骨架和亲锂材料组成,前者可以通过减小局部电流密度,延缓枝晶的产生,能缓解电池循环过程中的体积效应,后者则可以对锂的沉积行为进行调控,进一步诱导锂的均匀沉积,减少枝晶和死锂的生成。
根据亲锂材料种类和作用的不同,又可将其细分为:①储锂活性位点[图6(a)],即易于和锂形成合金的材料(如锌、银、硅、锡单质及其化合物等),形成的合金作为“缓冲层”可以降低异种材料之间的晶格失配度,进而减小锂在亲锂种子上的形核过电势,在骨架中均匀分布的亲锂种子可以确保锂的有序沉积,此外,镁、铝、钙、镧等单质及其化合物作为亲锂种子时,与骨架之间会形成凹陷“台阶”,锂在这些区域的临界形核体积和形核功较小,能有效提升锂的形核能力[图6(b)、(c)]。例如,在炭化的天然木头中引入氧化镁,制备的骨架材料可以在15 mA/cm2的超大电流密度下稳定循环,平均库仑效率大于96%,且无明显的枝晶产生[图6(d)、(e)]。
②杂化功能位点[图6(f)]。除了亲锂性功能之外,骨架通过物理和化学处理(掺杂、表面修饰、复合),也可以被赋予额外的功能,例如通过氟化处理可以在碳骨架中引入氟元素[图6(g)],这些氟元素可以进一步参与到沉积锂表面SEI膜的形成,构筑富含氟化锂的 SEI 膜[77-79]。这类功能化的骨架设计不仅为氟化SEI膜的构筑提供了新思路,也进一步丰富了骨架在锂负极中的作用。利用这些骨架可以有效地调控锂的均匀沉积和脱出,从而在一定程度上减少死锂的产生。
3.2 人工界面保护层
近期研究显示SEI膜中的聚合物组分在电解液中存在溶胀行为,溶胀后SEI膜的力学性能和离子传导能力都会发生变化,且随着电解液持续的溶剂化作用,SEI膜会逐渐被溶解,引起金属锂的暴露和腐蚀,活性锂转变为死锂。因而,相较于原生SEI膜,引入人工保护层,或者是通过电解液优化设计构筑高稳定性的SEI膜,有望抑制锂腐蚀和死锂形成,同时保证锂离子的均匀传输。
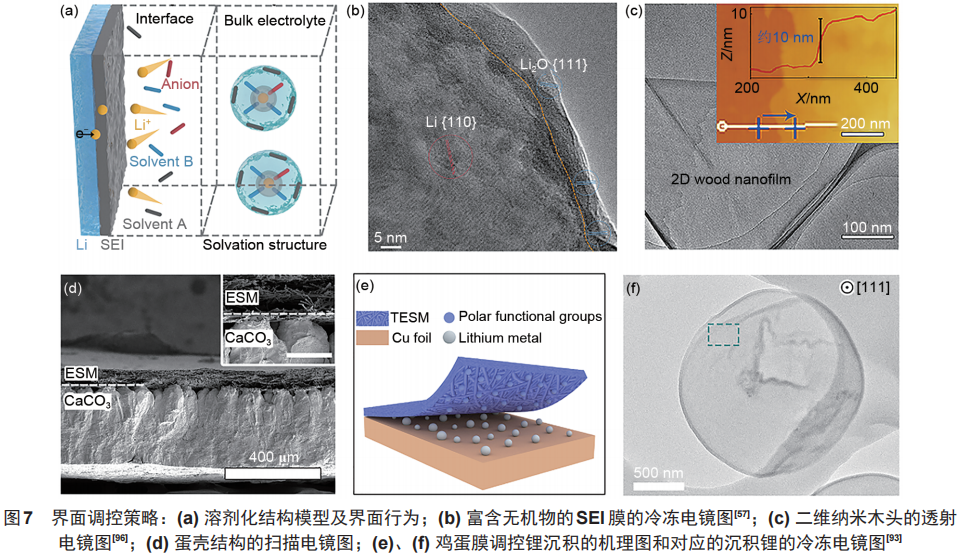
通过调控锂离子溶剂化结构可以有效改变SEI膜的结构组成,例如通过引入弱溶剂化的溶剂分子(如DOL),减弱其与锂离子的相互作用,使得更多的阴离子参与SEI膜形成,则可以构筑富含无机物的稳定SEI膜,实现锂的均匀沉积,并抑制锂腐蚀的发生[图7(a)、(b)]。
就人工保护层而言,构筑的保护层应当具有较低的溶解度、较高的离子传导能力、良好的黏附性和还原稳定性,可以有效地隔离锂和电解液。化工合成的聚合物膜(如聚环氧乙烯、聚偏氟乙烯)、无机钝化层、天然高分子材料及其复合物都是潜在的保护层。如一些自支撑结构的天然生物质薄膜(二维木头纳米片、蛋白质膜、竹纤维薄膜等),利用这些材料天然的表面化学性质和结构,可以促进锂离子均匀沉积,构筑稳定的 SEI 膜,并对枝晶生长发挥抑制作用,也可以在一定程度上减少死锂的积累[图7(c)~(f)]。
3.3 死锂激活策略
死锂的产生和积累难以被杜绝,因而发展有效的策略对生成的死锂进行处理具有重要意义。如前所述,死锂由失效的SEI膜空壳和金属锂碎片组成,深入研究还发现SEI膜中含有大量不导电的纳米氧化锂,正是这些纳米颗粒阻断了金属锂碎片与电极的电子通路。为了消除失效的SEI膜空壳,可以引入一些具有反应活性的物质。例如以碘介体作为一种“死锂激活剂”,可以在电池循环过程中自发地与“死锂”中的金属锂碎片和SEI膜中的Li2O发生反应,将死锂转化为可溶的碘化锂和碘酸锂[图8(a)];作为“锂载体”的碘化锂可以在浓度梯度的作用下迁移到正极侧,并被处于充电态的正极材料氧化,锂离子被回收存储;O18同位素示踪实验证实碘酸锂作为“氧载体”可将死锂中的Li2O搬运到活性锂表面,并对其进行有效的钝化保护。
这种策略不仅较好地解决了“死锂”难题,实现其再生利用,而且降低了电池产气量,并抑制了枝晶生长,电池循环稳定性显著提升。将碘介体制备成缓释的碳胶囊,可在纽扣全电池和0.5Ah级软包电池中得到进一步的应用[图8(b)、(c)]。除了碘单质,金属碘化物亦可作为死锂激活剂,并且可以在一定程度上缓解碘介体对锂的腐蚀[图8(d)、(e)];此外,死锂激活策略在固态聚合物金属锂电池中同样适用,可大幅提升电池寿命和循环稳定性。
3.4 固态电池体系中的死锂抑制
在固态电池体系中,同样存在死锂问题。无论是聚合物电解质体系或者是无机固态电解质,由于锂与电解质之间的副反应,界面上会产生大量离子导电性差而电子导电性较好的副产物,严重恶化界面的稳定性和离子传输能力,降低电池的充放电效率,诱发活性锂损失。此外,由于固态电解质富含晶界,且自身具有一定的电子导电性,锂离子会在晶界处直接沉积,诱发枝晶生长,造成活性锂损失和电池短路。
通过电解质工程优化可以缓解固态电池体系中死锂的生成和积累:①外源性导电界面构筑。在界面上沉积高导电、高导锂的材料,例如铂、金、银等,为死锂提供外源性的导电路径[图9(a)、(b)],使得死锂可以在一定程度上被重新利用。②晶界修饰。例如,向无机Li4SnS4电解质晶界处引入电子绝缘而离子导电的碘化锂,同时对电解质界面及体相晶界进行稳定化,有效抑制枝晶沿着晶界生长,减少死锂的产生,电池循环寿命可提升25倍[图9(c)、(d)]。